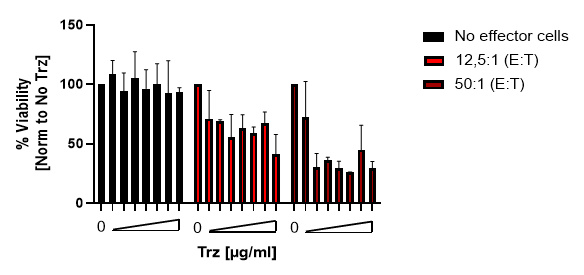
NK Cell Killing Assays (ADCC Assay)
NK cells can recognize and kill cells that show signs of oncogenic transformation. This unique functionality puts NK cells in the center of immunotherapy research seeking to help NK cells identify tumor cells and enhance the therapeutic activity of NK cells.
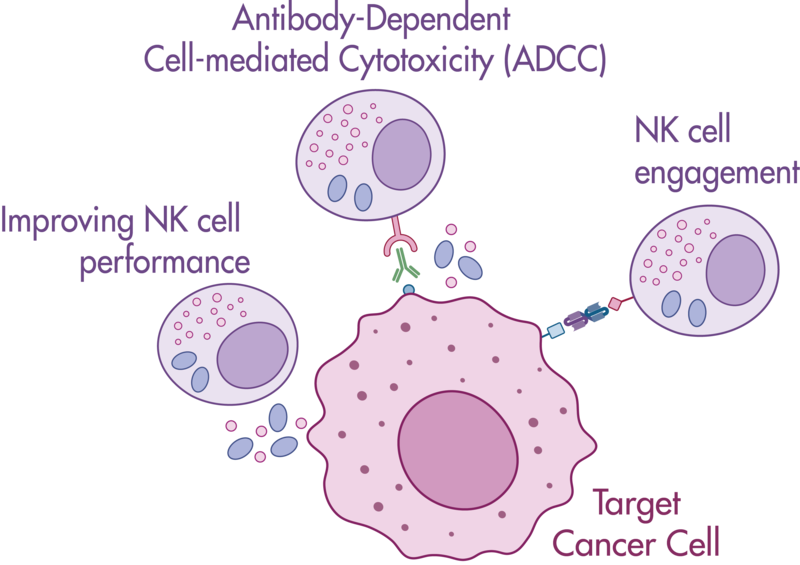
Natural killer cells are regulated by activating and inhibiting receptors. Tumor cells express stress-induced ligands or lack sufficient MHC-I molecules, which are recognized by NK cells prompting the release of cytotoxic granules. The innate immune process antibody-induced cellular cytotoxicity (ADCC) also involves NK cells. They express Fc receptors on their surface, recognizing and killing antibody-coated target cells. Many advantages of NK cell therapies are based on their innate nature; for example, cells can be transferred between individuals, opening opportunities for off-the-shelf products.
Reaction Biology offers a variety of immune cell assays. Our ADCC assays enable the investigation of antibody treatment sought to recognize malignant cells and label them for killing by NK cells. Get in contact with us to have a conversation with our scientists about your research goals and how we can help you achieve them.
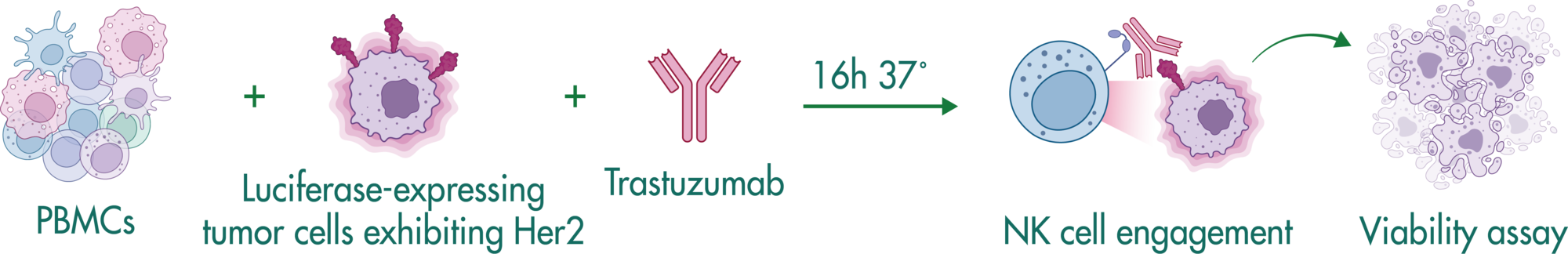
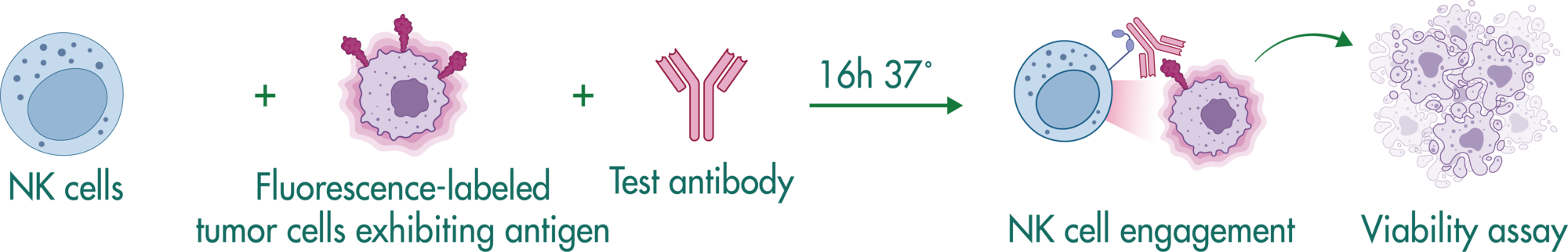
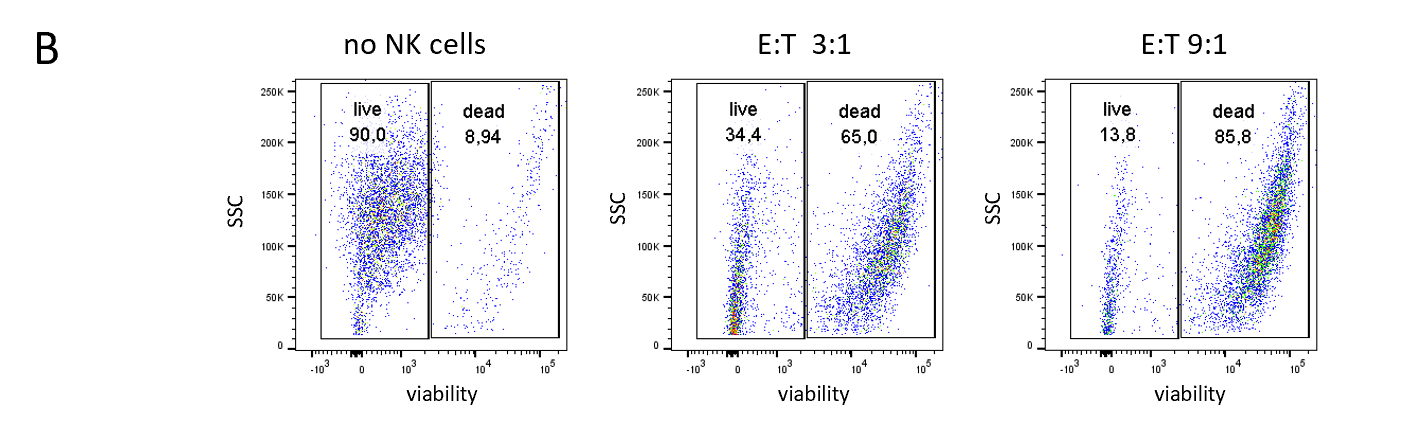
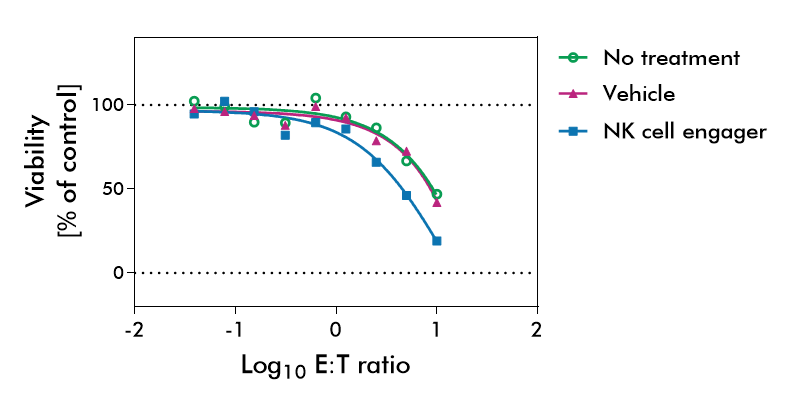
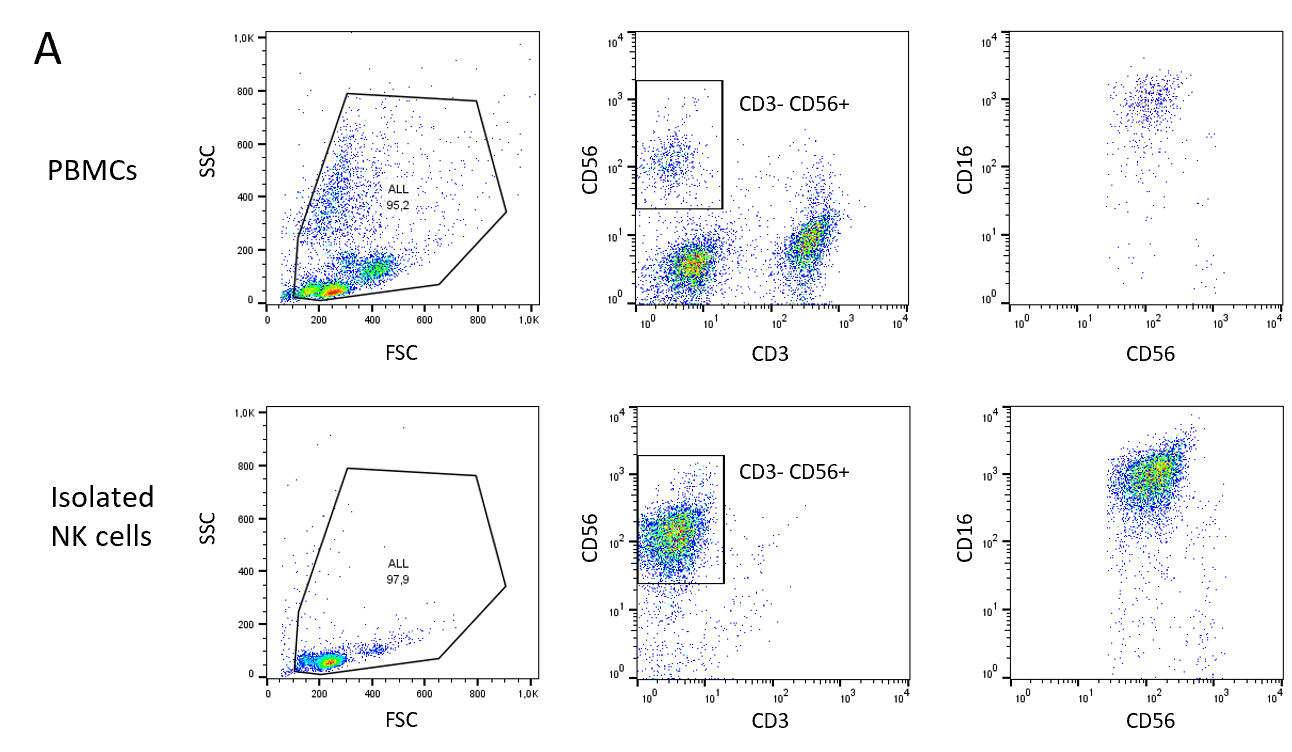 NK cells were isolated from healthy donor PBMCs via an NK Cell Isolation Kit. The purity of isolated NK cells was quantified by staining with NK cell-specific markers CD3-, CD56+, CD16+ before isolation (upper row) and after isolation (lower row). 10 million NK cells could be recovered from 300 million PBMCs with 88% purity.
NK cells were isolated from healthy donor PBMCs via an NK Cell Isolation Kit. The purity of isolated NK cells was quantified by staining with NK cell-specific markers CD3-, CD56+, CD16+ before isolation (upper row) and after isolation (lower row). 10 million NK cells could be recovered from 300 million PBMCs with 88% purity.