Apoptosis Assay Services for Drug Discovery
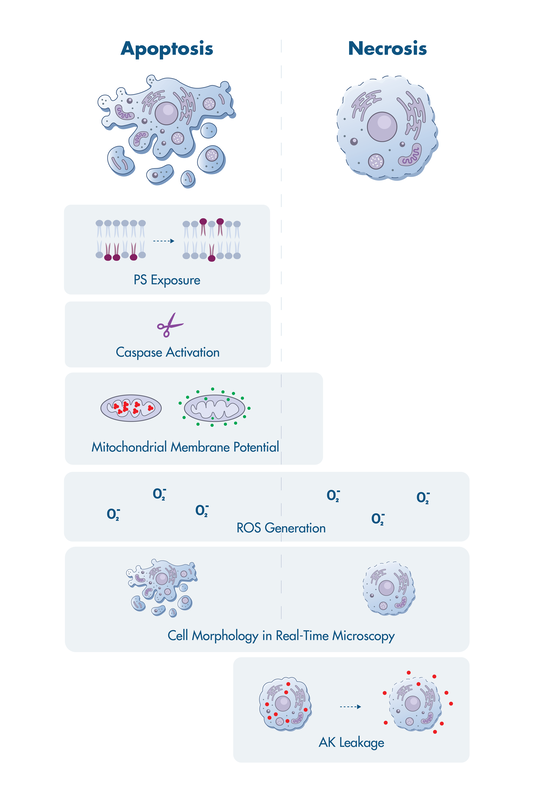
Apoptosis assays elucidate the cell death-related events during tumor cell killing by anti-cancer drugs. Two key questions need to be answered: Do tumor cells die? And, through which pathway do the cells die?
Reaction Biology offers a suite of cell death assay services to investigate events that have been linked to apoptosis including activation of mitochondrial membrane permeabilization, caspase activation, surface exposure of phosphatidylserine, and more.
Apoptosis assays are available for single and multi-time point analysis as well as real-time live-cell imaging.