CAR T-Cell Therapy Platform
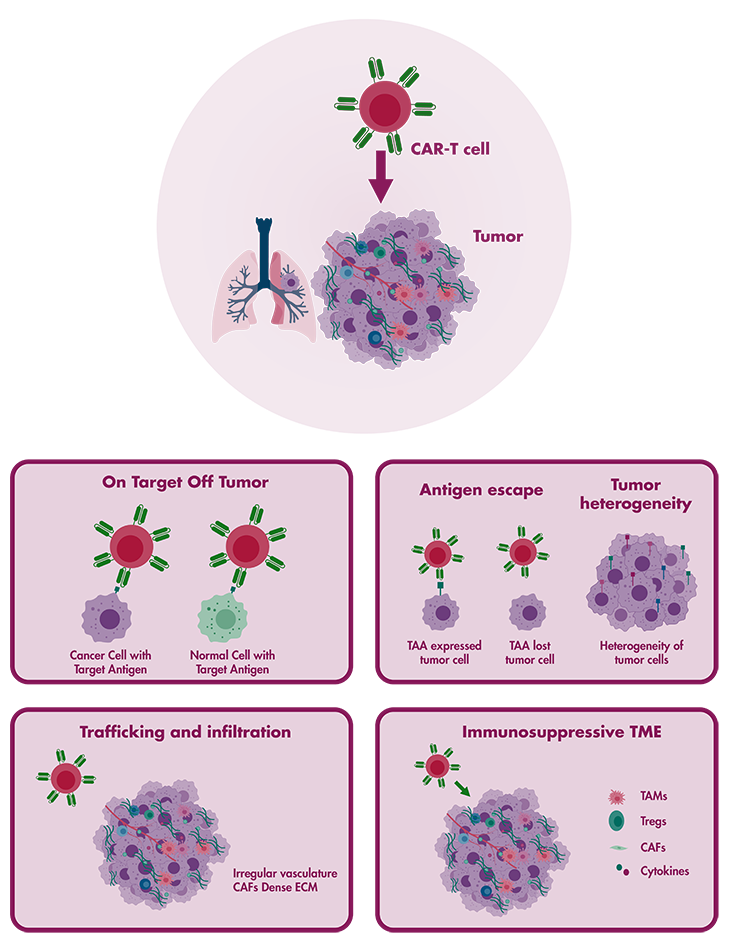
CAR T-cell therapy, especially when directed against CD19 for B cell cancers, has demonstrated remarkable efficacy and received FDA approval in 2017. Nonetheless, despite its achievements, there exist challenges that must be addressed for CAR T-cells to realize their complete therapeutic potential against both solid tumors and haematological malignancies. Reaction Biology stands at the forefront of preclinical cancer immunotherapy, providing a wide array of CAR T-cell development services. Our dedicated platform, crafted by our team of immuno-oncology specialists, employs a blend of in vitro and in vivo studies to accelerate your research endeavors.
We offer a range of services, including:
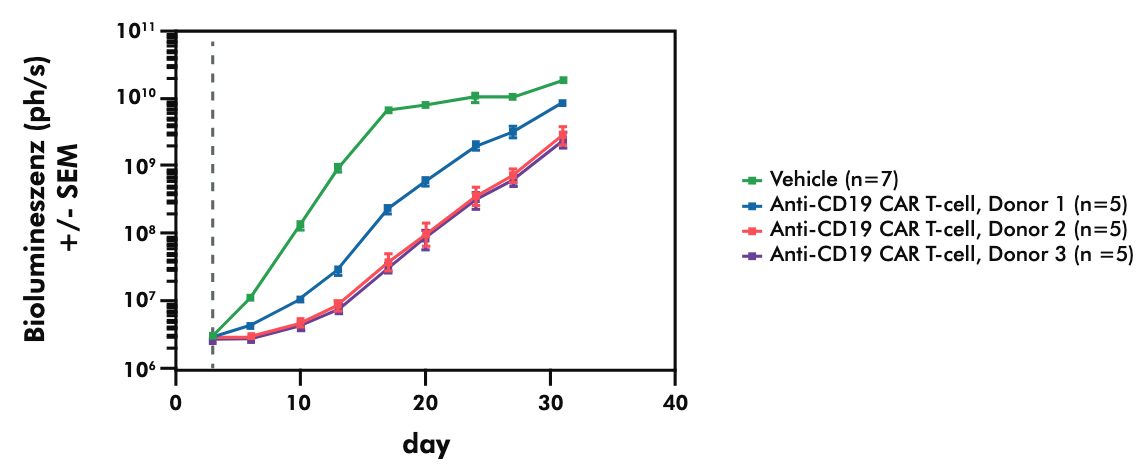
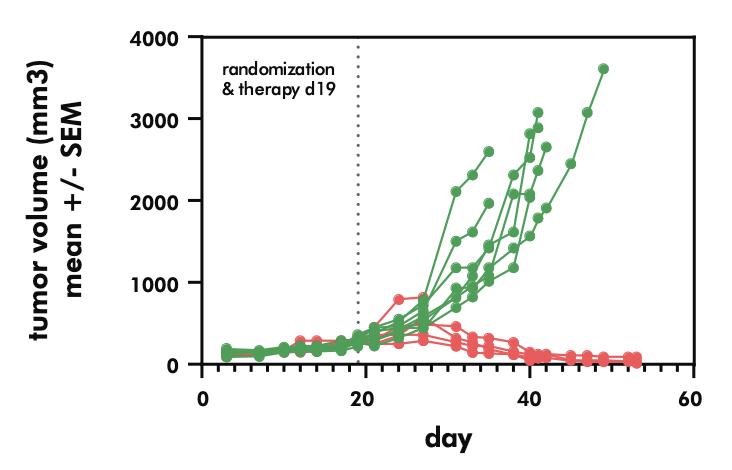
- Evaluating the potency and pharmacokinetics of your CAR T candidate within in vivo models of cancer biology
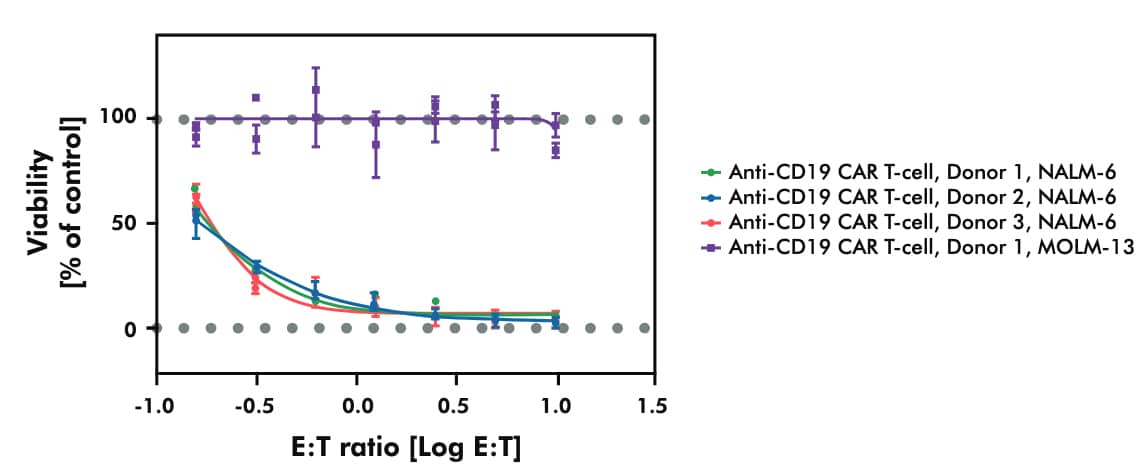
- Assessing the in vitro cytotoxicity of your CAR T candidate across established cancer cell lines
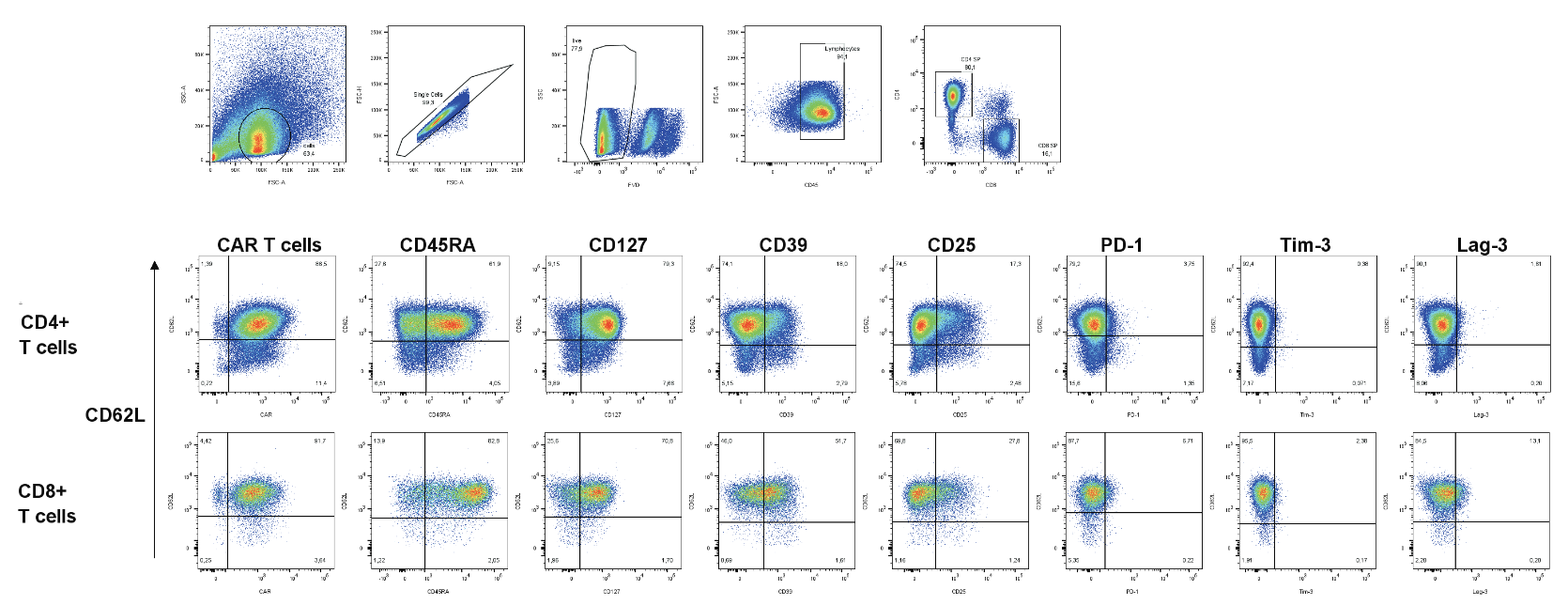
- Delving into the activation and memory status of CAR T-cells to illuminate their therapeutic potential
- Testing the efficacy of CAR T candidates in xenograft models of solid tumors
With our platform’s capabilities, we empower you to refine and advance your therapeutic strategies, driving forward progress in cancer immunotherapy.