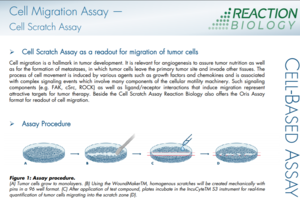
Cell Migration Assays (Wound Healing Assay)
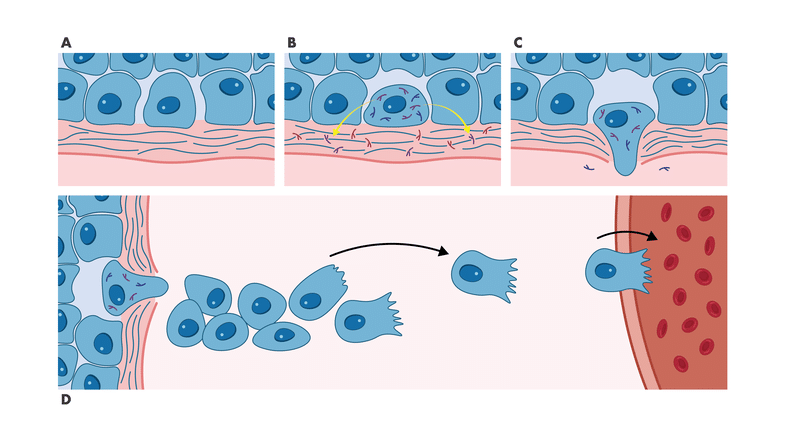
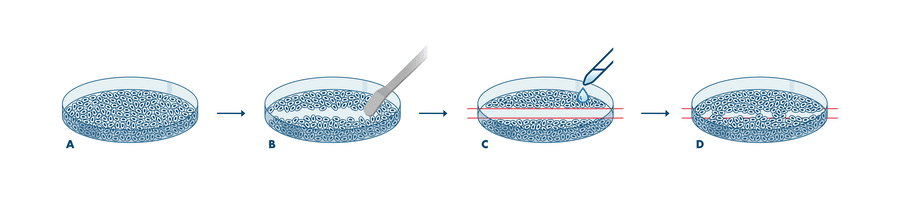
Cell migration assays are essential tools for the investigation of tumor cell metastasis. The Wound Healing Assay (also known as Scratch Assay) measures how quickly cells close a defined gap in a confluent monolayer through undirected migration, providing a functional readout of compound effects on cell motility.
Cell migration is a hallmark of cancer progression and metastatic disease. It enables angiogenesis to support tumor growth and drives metastasis, where tumor cells leave the primary site and invade distant tissues. This migratory process is triggered by growth factors, chemokines, and other stimuli, activating complex signaling cascades involving key components of the cellular motility machinery. Druggable targets such as FAK, cSrc, and ROCK, along with the ligand/receptor interactions that initiate migration, are promising opportunities for anti-metastatic drug development.
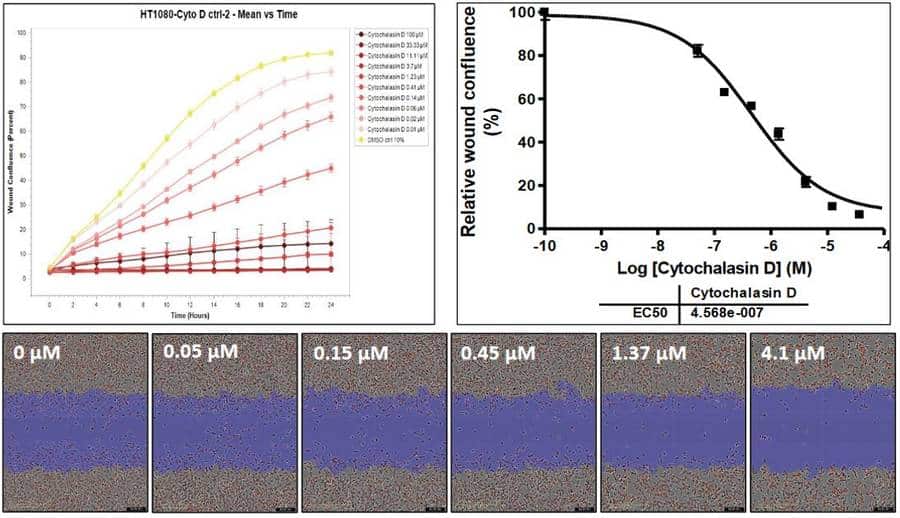
The Wound Healing Assay enables functional characterization of both migration inhibitors and promoters, supporting a range of high-throughput and combination treatment applications:
- Target validation: Confirm the role of specific signaling pathways (e.g., FAK, Src, Rho/ROCK) in tumor cell motility
- Compound screening: Evaluate anti-migratory potency of small molecules, biologics, or combination treatments in high-throughput formats