PDE Assay Services
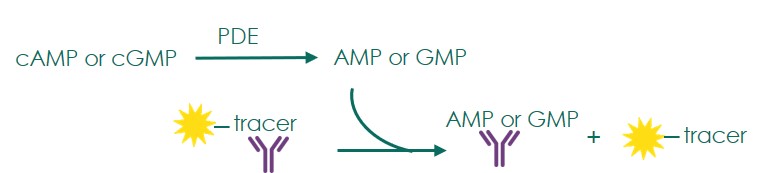
Phosphodiesterase (PDE) screening is performed with an activity assay format based on the hydrolysis of cyclic AMP and cyclic GMP. Inhibition of PDEs prevents the regulation of these second messengers and was found useful in the treatment of a variety of conditions including pulmonary hypertension, acute refractory cardiac failure, erectile dysfunction, etc.
- The activity of PDEs is measured with the Transcreener AMP2/GMP2 FP PDE assay platform (BellBrook labs)
- Low and large scale screening, as well as high-throughput PDE screening options, are available
- Custom-tailored PDE assay development possible
At Reaction Biology, we offer PDE screening services for pharma, biotech, and academic institutions with a commitment to quality data and quality service. Our global business development team is available to discuss your PDE assay needs with you today.