Combination therapy to enhance BiTE-induced T cell killing of luciferase-labeled B-cell lymphoma cell line OCI-LY1
Assay principle
Bi-specific antibodies contain two binding sites to engage T cells and tumor cells resulting in tumor cell killing by (i) bringing the cells in close proximity, and (ii) stimulating cytokine release in T cells.
In this study example, we determine the capacity of a bi-specific engaging molecule to facilitate tumor cell killing by T cells and the combinatorial potential with other clinically relevant agents.
Setup
CD19-expressing B cell lymphoma cells stably expressing firefly luciferase are co-cultured with PBMCs at an E:T (effector to target cell ratio) of 2:1. We tested the bi-specific T cell engager Blinatumomab recognizing CD19 and CD3 at different concentrations (7x semi-log), with or without additional compounds. The co-culture was incubated for 4 days, after which the viability of the tumor cells was measured by luciferase activity.
Study results
BiTE Blinatumomab and the small molecule Lenalidomide act synergistically to kill CD19-positive B cell lymphoma cells, whereas the combination with S63845 acts antagonistically.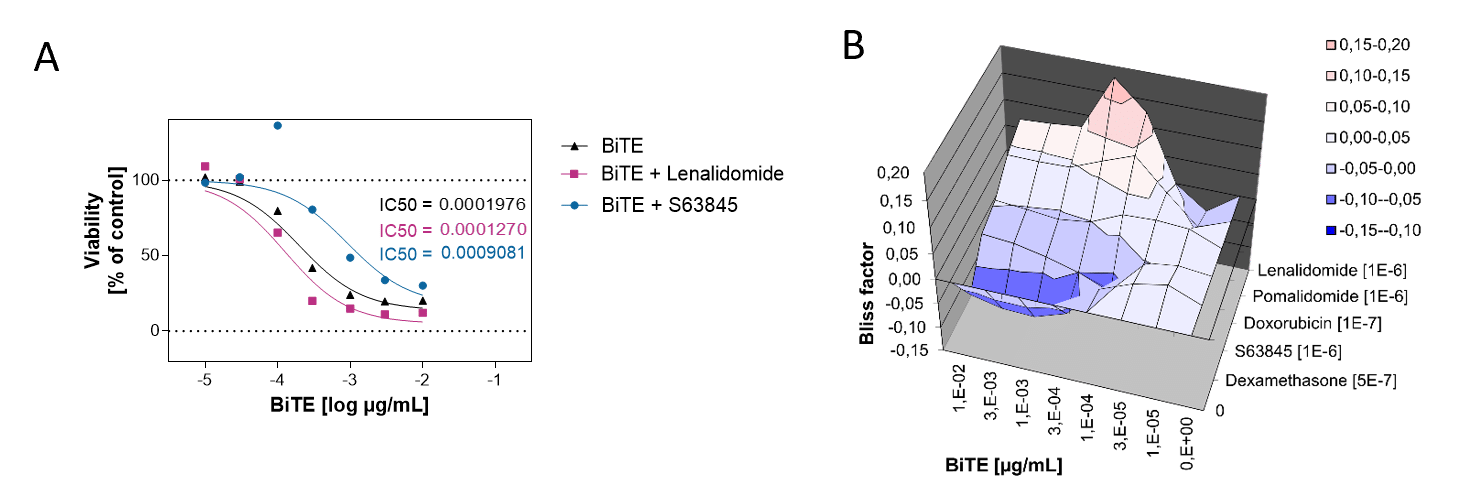
A. PBMCs from a healthy donor were incubated with the B cell lymphoma cell line OCI-LY1 at a 2:1 E:T ratio and treated with a range of 7 concentrations of Blinatumomab. Luciferase expression of live tumor cells was measured after 4 days, and viability was calculated using Staurosporine treated cells as maximal cell death control and untreated target cells as maximal viability control. An IC50 of 0,19 ng/ml was found for Blinatumomab. The combinatorial effects of Blinatumomab with Lenalidomide and MCL1-inhibitor S63845 are shown as examples.
B. Using the bliss factor scoring, we determined which drug combinations produce additive, synergistic, or antagonistic effects for killing tumor cells.