ITC Assay Service for Drug Discovery
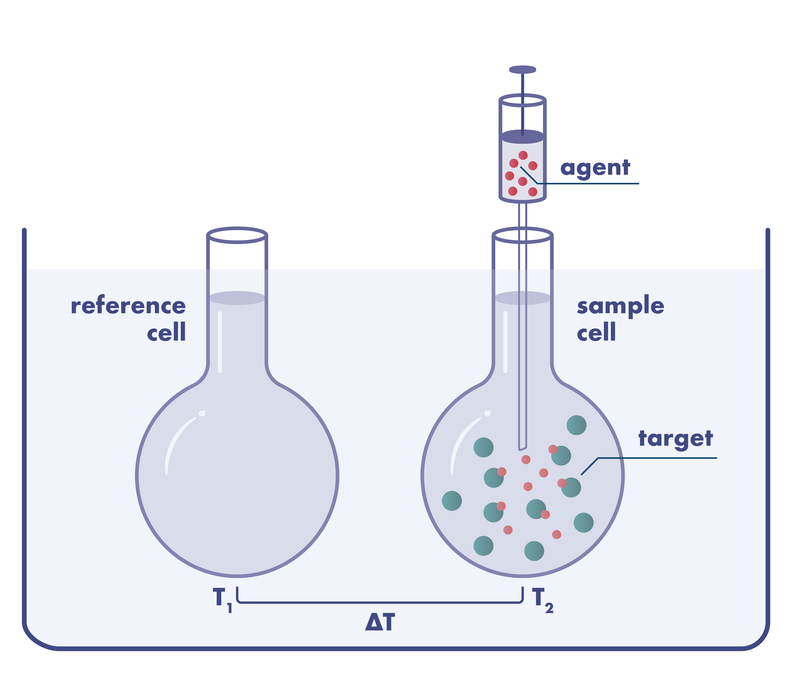
Isothermal titration calorimetry assays (ITC assays) are used in the drug discovery process to determine the thermodynamic parameters of a compound-ligand interaction in solution. Isothermal titration calorimetry is based on the measurement of the heat released or absorbed as the result of a chemical reaction such as the binding event of a compound to its ligand.
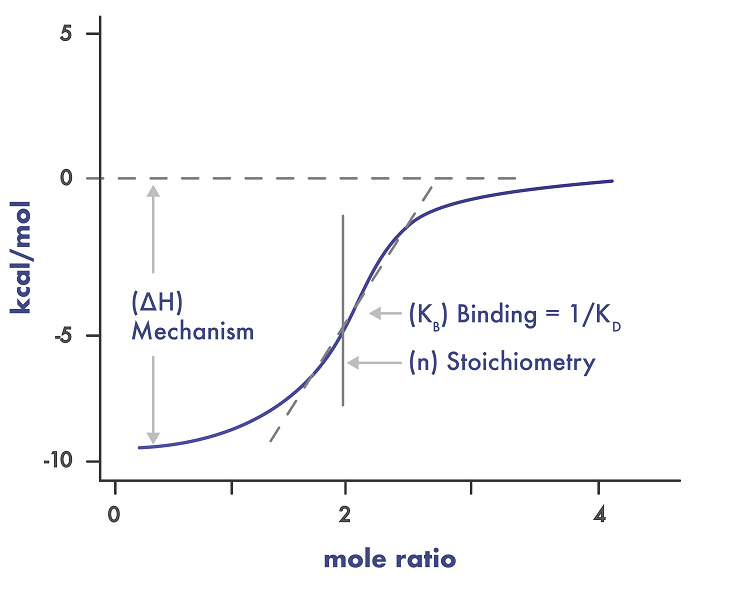
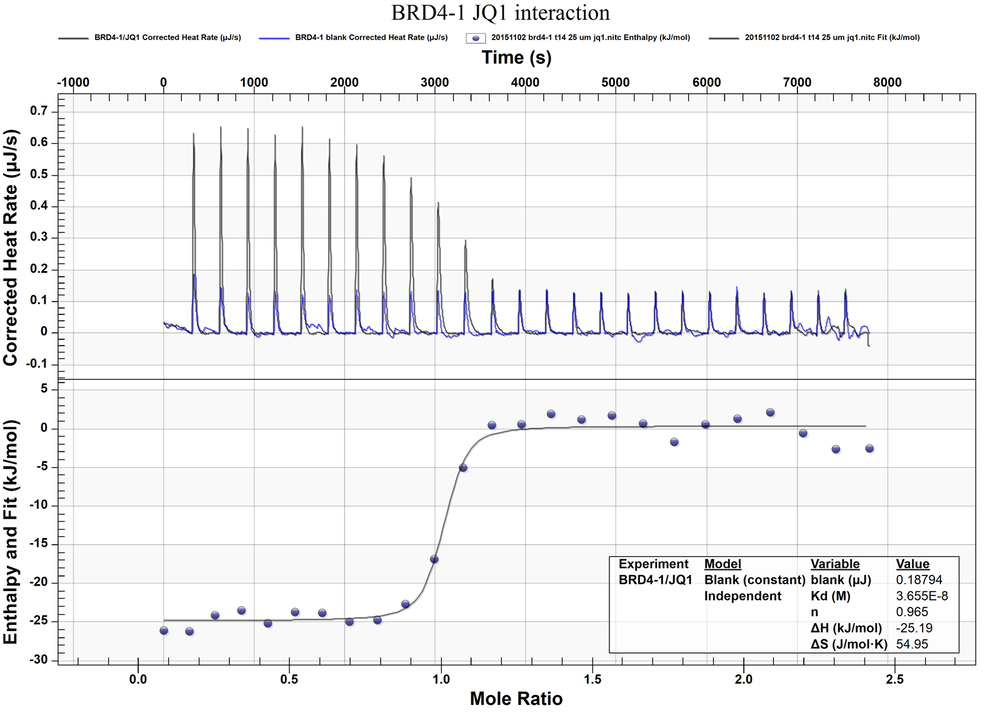
During the ITC screening, one component of the reaction is contained in a temperature-controlled cell, then the binding partner is gradually titrated into the system. When binding occurs, heat is either released or absorbed. This heat change is directly proportional to the amount of binding. This technique is suitable as a secondary screen yielding not only the binding affinity but also the thermodynamic profile of a ligand-target interaction giving insight into the structure-function relationship on the molecular level.
- Label-free technique avoiding any influences of labels or immobilization on the binding reaction
- Deliverables: binding stoichiometry, binding enthalpy (∆H), entropy (∆S), binding affinity KD