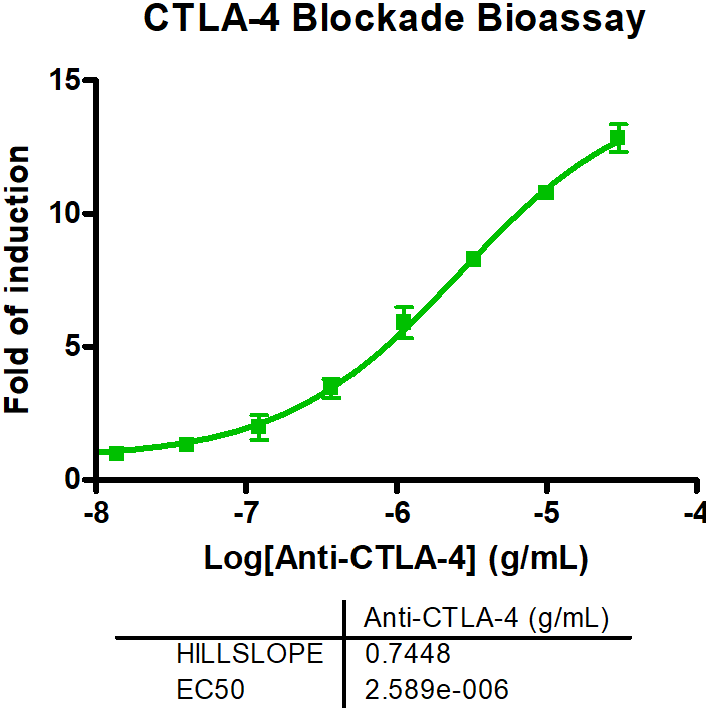
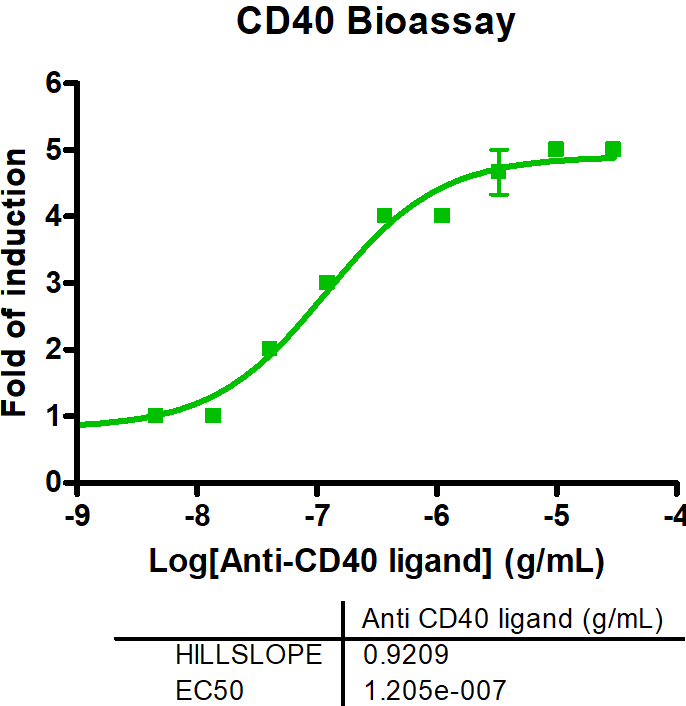
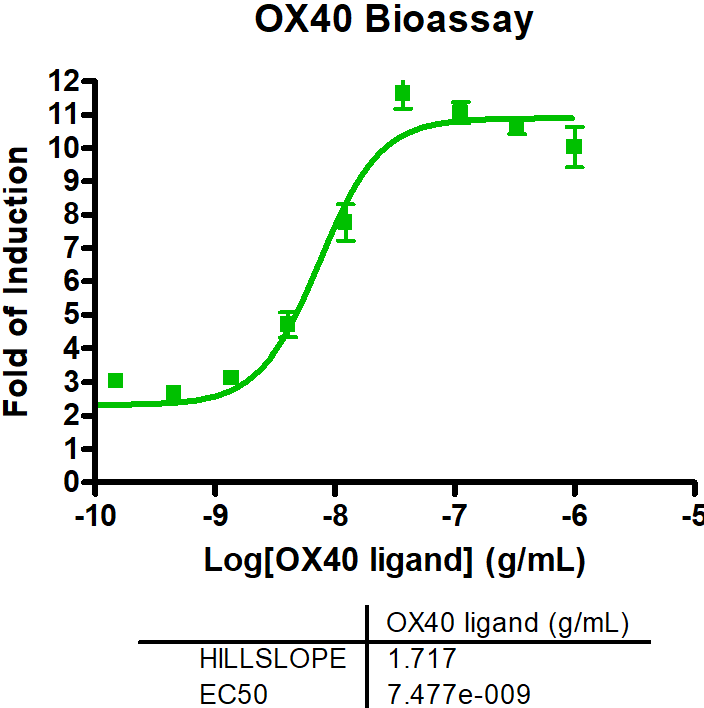
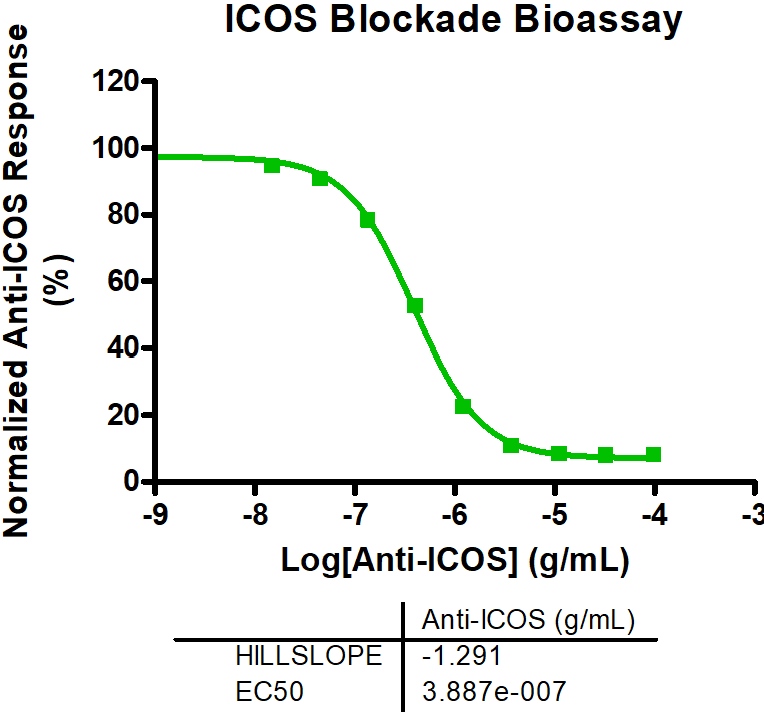
Immune Checkpoint Assays
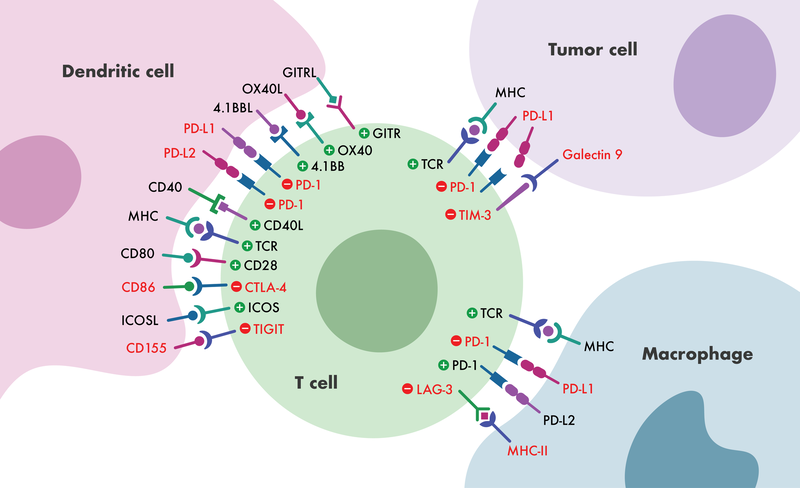
Immune checkpoint molecules play an important role in the elimination of cells that express foreign antigens while maintaining tolerance to self-antigens. Given their capacity to regulate immune responses, immune checkpoint receptors have emerged as promising new immunotherapy targets for a variety of diseases, including cancer and autoimmune disorders.
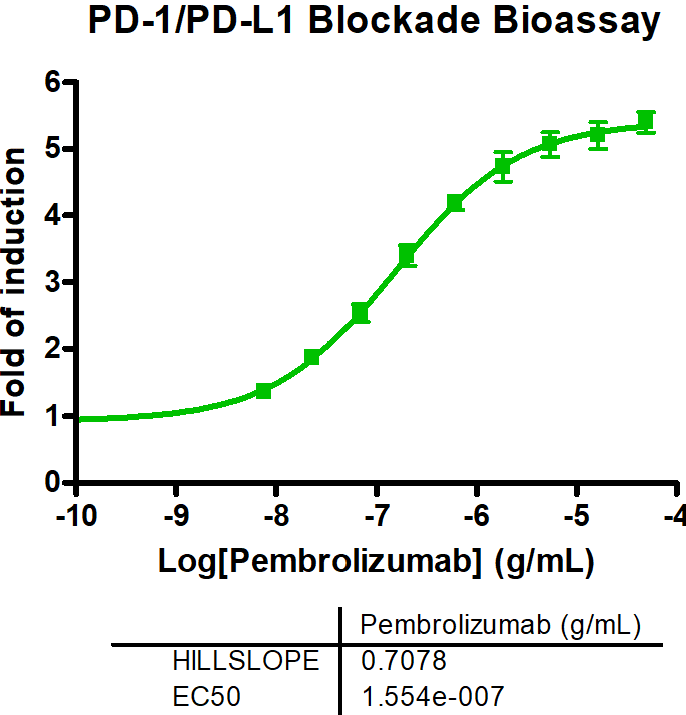
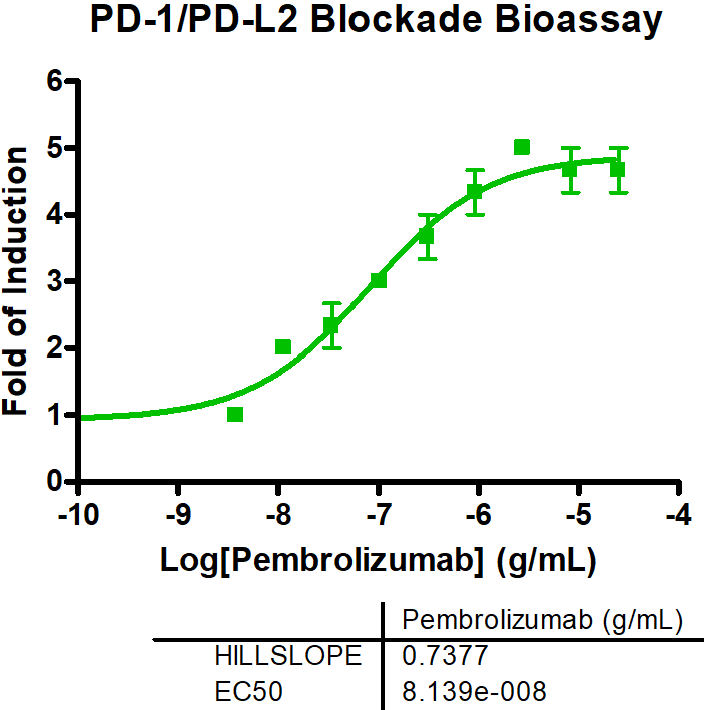
In general, immune checkpoints are divided into two categories: activating receptors and inhibitory receptors. The two key mechanisms through which an immunotherapeutic compound or biologic acts are either by blocking the immune pathway (antagonists) or by enhancing the immune pathway response (agonists). Using novel therapeutics to target these immune response pathways has proven to be an effective treatment strategy against multiple diseases. Reaction Biology’s scientists have developed a comprehensive portfolio of immune checkpoint assays, ready to be used in a variety of settings, from the screening and characterization of biologics (antibodies) in cell-based assay formats to quantifying potency and binding of small and large molecules in biochemical assay formats.
Please reach out today to discuss how we can best assist you with your immuno-therapy research.