Scientists at Reaction Biology have developed a comprehensive RAS drug discovery assay suite to help accelerate drug discovery projects targeting the KRAS/MAPK pathway. The comprehensive assay suite includes multi-spectrum assay tools and techniques for studying and characterizing novel KRAS pathway inhibitors in a variety of assay formats, including biochemical and biophysical assays and RAS-targeting compound screening in intact cells using NanoBRET™ Technology.

- Biochemical Assay Services
- Biophysical Assay Services
- Cell-Based Assays
- In Vivo RAS Models
- Recombinant Proteins
- Custom Tailored Assay Services
Biochemical and Biophysical RAS Assay Details
RAS::SOS1 Protein:Protein Interaction (PPI) Assay
Disruption of SOS1 binding to KRAS can be used as an orthogonal method for studying SOS1 specific compounds. The assay uses an HTRF-based detection of interaction.
cRAF recognizes the GTP-bound form of RAS. cRAF binding assay can be used for the identification of disruptors of interaction between Ras and cRAF, as well as quantification of the nucleotide exchange reaction. This assay can be used as an alternative to the regular Nucleotide Exchange Assay with an optional examination of SOS1 independent GTP binding. The assay uses an HTRF-based detection of interaction.
Please inquire about custom-tailored GTPase assay development.
The PPI assay is available for wild-type RAS and various RAS G12 mutants.
The RAS NEA allows the monitoring of SOS1/2 mediated exchange of fluorescently labeled GDP to GTP.
The main application of the assay is to identify compounds that lock RAS in the inactive “OFF” state by preventing GTP binding.
An alternative NEA format utilizes GTP labelled with DY-647P1 and monitors the increase in HTRF signal observed upon GTP binding to RAS. The assay is performed at lower GTP concentrations compared to the standard RAS NEA and can evaluate various modes of nucleotide exchange inhibition.
Please inquire about custom-tailored assay development.
The RAS NEA is available for wild-type RAS and various G12 mutants thereof.
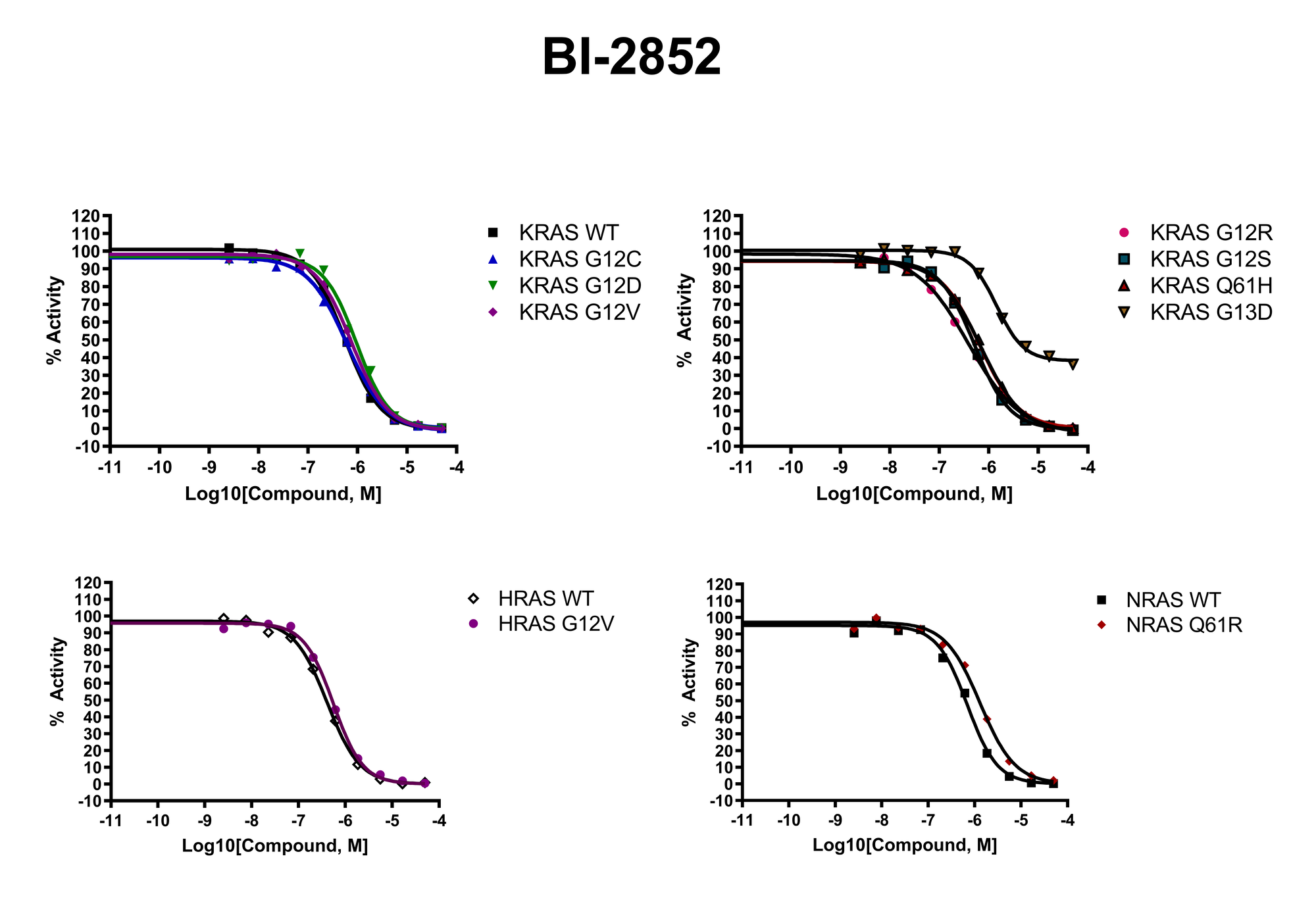
The KRAS mutants NEA (Nucleotide Exchange Assay) selectivity panel is designed for screening and profiling of potent inhibitors against KRAS mutants.
The HTRF (Homogeneous Time Resolved Fluorescence) based NEA assay employs GTP labeled with DY-647P1 and monitors the increase in HTRF signal observed upon GTP binding to RAS. The assay can evaluate various modes of nucleotide exchange inhibition.
The primary goal of the panel is to help scientists evaluate compound specificity between various KRAS mutants and the other two RAS homologs.
The following proteins are currently included in the panel:
| KRAS WT | KRAS G12R | HRAS WT |
| KRAS G12C | KRAS G12S | HRAS G12V |
| KRAS G12D | KRAS Q61H | NRAS WT |
| KRAS G12V | KRAS G13D | NRAS Q61R |
The panel will run on a monthly basis.
Please inquire about custom-tailored assay development.

A schematic showing the specificity of two compounds, MRTX-849 and BI-2852, against various KRAS mutants and two RAS homologs.

Thermal Shift Assays (TSAs) are used to assess the effects of compounds on protein stability. Selectivity of two compounds KRAS mutant G12C is shown.

The TSA is available for RAS and various G12 mutants thereof as well as SOS1 and SOS2. Please inquire about custom-tailored assay development.
Surface Plasmon Resonance (SPR) is used to quantify the binding affinity of the molecule as well as binding kinetics. A comparison between KRAS WT and mutant proteins can be performed to determine selectivity.
KRAS and various G12 mutants thereof as well as SOS1 are established for SPR analysis. Please inquire for custom-tailored assay development.


Example study: The KRAS G12D mutant selective peptide KRpep-2d was used to show the difference in the binding of KRpep-2d to mutant G12D versus wild type KRAS and other mutants. The peptide binds to all targets, however, the binding affinity (KD) of the peptide is 15 times higher when interacting with the G12D mutant.
Testing compound binding to the target in the physiologic environment of intact cells is the ideal assay to bridge from biochemical to phenotypic compound testing in cellular tumor models.
Advantages of the NanoBRET Target Engagement RAS Assay:
- Testing of compound-target binding in intact cells
- Determination of binding affinity and target protein occupancy as well as residence time in the intracellular environment
- A variety of orthosteric and allosteric inhibitors to RAS can be tested
- Multiwell assay suitable for medium-throughput upscaling
- High reproducibility
RAS-Related Assays List
* Please inquire
NEA … Nucleotide Exchange Assay
PPI … Protein:Protein Interaction
SPR … Surface Plasmon Resonance
TSA … Thermal Shift Assay
Why Choose Reaction Biology for Assay Research
We’re committed to providing quality service throughout our partnership with you. From start to finish, the business development manager assigned to you will be just a phone call away. This ensures the high-quality of data through robust and reproducible assay formats for our RAS assay suite.


